Innovative Pre-clinical Research
Synovo is a drug discovery company with a primary focus on inflammation and innate immune processes.
We are located in Tübingen, Germany which is both a major centre for basic research and also home to a vibrant bio-medical start-up scene.
We have two main business areas:
- we provide drug discovery services in pharmacology, medicinal chemistry and bioanalytics, and,
- we develop in-house novel therapeutics and related diagnostics
Our culture is focused on generating insight through inter-disciplinary, collaborative work with clients and partners.
Within our team of nearly 50 people we have specialists in synthetic chemistry, pharmacology, analytics, immunology, neurology and biochemistry. In addition, we have a broad network of research partners throughout the world collaborating with us in areas like basic disease mechanisms, drug formulation technology, surface modification and drug mode of action.
We welcome enquiries and we are proud of our reputation for helping colleagues and partners achieve their research goals.
What we do
PHARMACEUTICAL SERVICES
Synovo offers the possibility of improving your pre-clinical data set. Take the opportunity to explore deeper parameters and learn more from your studies by adding our services to your projects.
Learn more about our
INNOVATION
We are committed to contribute to health improvement by developing novel therapeutic options for global unmet needs.
Learn more about our
TEAM
The Synovo family shares a common interest toward developing science-driven mind changing ideas, by interdisciplinary and creative team work. Moreover we are aware of our place in the local community, trying to exemplify an active lifestyle.
Learn more about our
Latest news
Novel class of Fumarate Esters
We at Synovo are happy to announce our latest publication in Inflammopharmacology and with it our new class of Fumarate Esters. This […]
Newsletter Subscription
Join our scientific community by signing up to this email newsletter and get monthly updates on our services, research and new products. […]
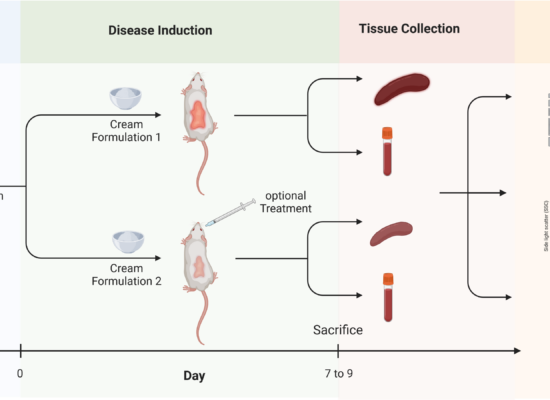
Isostearic acid and its ability to induce psoriaform lesions in murine model
We would like to share with you our latest publication and discovery on the topic of the cream-induced psoriasis murine model. In […]